When it comes to organic synthesis, certain reactions stand out for their versatility and utility. One such reaction that has garnered attention is the thiol attacking alkene process. This fascinating transformation involves a sulfur-containing compound, known as a thiol, interacting with an alkene—a molecule defined by its carbon-carbon double bond. The result? A unique array of products that can serve various purposes in chemical research and industry.
With its intriguing mechanism and broad applications, understanding thiol attacking alkenes opens doors to innovative solutions in synthetic chemistry. Whether you’re a seasoned chemist or just beginning your journey into organic synthesis, this topic will illuminate key concepts that are essential for mastering modern chemical transformations. Buckle up as we explore the ins and outs of this remarkable reaction!
The Mechanism of Thiol Attacking Alkenes
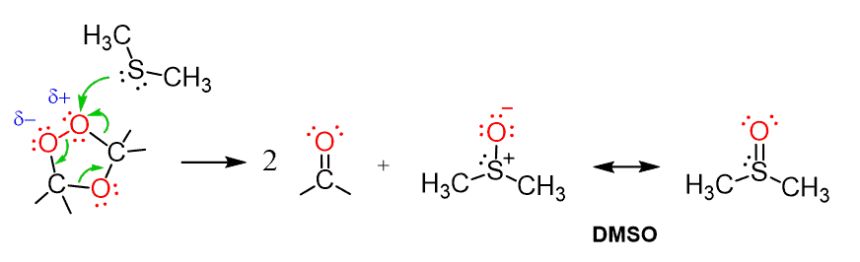
The mechanism of thiol attacking alkenes is a fascinating process. It begins with the nucleophilic nature of thiols, where the sulfur atom donates a pair of electrons.
Once in proximity to an alkene, this electron-rich site targets the electrophilic carbon-carbon double bond. The alkene’s π-bond breaks, leading to the formation of a new sigma bond between the sulfur and one carbon atom.
This reaction typically follows a two-step pathway: first, forming a cyclic intermediate known as a thioether or mercaptan. Afterward, proton transfer occurs to stabilize that intermediate fully.
This elegant interaction transforms both reactants into products while creating valuable compounds for further applications in organic chemistry. Understanding this mechanism opens doors for optimizing reactions and developing innovative synthesis strategies.
Applications of Thiol Attacking Alkenes in Organic Synthesis
Thiol attacking alkenes play a pivotal role in organic synthesis, especially in the formation of valuable sulfur-containing compounds. These reactions offer an efficient pathway to construct thioethers and thiol esters, which are key intermediates in various chemical processes.
One significant application is in drug development. Thiol adducts derived from alkenes can enhance the biological activity of pharmaceutical agents. Researchers leverage these transformations to create more effective therapeutics with improved properties.
Moreover, thiol attacking alkene reactions are essential for synthesizing natural products. Many biologically active molecules contain sulfur functionalities that arise from such transformations. This makes them invaluable tools for chemists aiming to replicate nature’s complexity.
The versatility of this reaction extends into polymer chemistry as well. By employing thiols on alkenes, scientists can design advanced materials with tailored characteristics for specific applications, including coatings and adhesives.
Advantages and Limitations of Thiol Attacking Alkenes
Thiol attacking alkenes offer several advantages that make them appealing in organic synthesis. One notable benefit is their ability to form stable thioether products. This stability enhances the overall yield of reactions, making thiol-alkene interactions valuable for chemists.
Moreover, thiols are generally more nucleophilic compared to other functional groups. Their reactivity allows for faster reaction rates, which can be crucial when time is a factor in research and development.
However, there are limitations to consider. The presence of sterically hindered alkenes may slow down or inhibit the reaction entirely. Additionally, some thiols can have strong odors or toxicity issues, necessitating careful handling and consideration during experiments.
Environmental factors also play a role; certain conditions may limit the effectiveness of thiol attacking processes. These challenges underscore the importance of context when employing this method in synthetic pathways.
Examples of Thiol Attacking Alkene Reactions in Literature
Numerous studies have documented the fascinating interactions between thiols and alkenes. One notable example is the reaction of thiophenol with various unsaturated compounds, showcasing its ability to form stable thioether products.
In another study, researchers demonstrated the regioselectivity of thiol attacking alkenes in generating valuable intermediates for pharmaceutical synthesis. These findings highlight how specific conditions can influence reaction pathways and product yields.
Additionally, a groundbreaking paper explored catalytic approaches that enhance the efficiency of these transformations. By using metal catalysts, scientists achieved remarkable rates and selectivities, expanding potential applications in organic chemistry.
Some literature even delves into green chemistry aspects by utilizing renewable resources as substrates for thiol-alkene reactions. This innovative approach not only reduces waste but also promotes sustainability within chemical processes.
These examples illustrate just a fraction of the rich landscape surrounding thiol attacking alkene reactions and their significance in modern organic synthesis.
Future Perspectives and Developments in Thiol Attacking Alkenes
The future of thiol attacking alkenes looks promising, with ongoing research focused on enhancing reaction conditions. Scientists are exploring new catalysts that could increase efficiency and selectivity, making these reactions more sustainable.
Advancements in green chemistry principles are also driving innovation. The integration of renewable resources into the synthesis process is becoming a priority, reducing environmental impact while maintaining yield.
Moreover, applications in pharmaceuticals and materials science continue to expand. Researchers are investigating novel thiol-based compounds for targeted drug delivery systems or developing new polymeric materials with improved properties.
Automation and machine learning technologies may revolutionize how these reactions are studied. Data-driven approaches can lead to faster discovery of optimal conditions and better understanding of reaction mechanisms.
As interdisciplinary collaborations grow, we can expect exciting breakthroughs that leverage insights from various fields to unlock further potential within thiol attacking alkene chemistry.
Conclusion
The role of thiol attacking alkenes in organic synthesis cannot be overstated. This reaction not only provides a pathway for the transformation of simple compounds into more complex structures, but it also plays a vital part in various applications across different fields. The mechanism involved is intricate yet fascinating, showcasing the beauty of chemistry at work.
While there are advantages to using thiol attacking alkenes—such as their efficiency and selectivity—limitations do exist. Researchers continue to address these challenges, exploring new methodologies and improving existing ones. The literature is rich with examples that highlight both successful reactions and innovative approaches to overcoming obstacles.
Looking ahead, the future of thiol attacking alkene research appears promising. As scientists delve deeper into this area, new discoveries may emerge that enhance our understanding and expand its utility even further. With ongoing advancements, we can anticipate exciting developments that will reshape how we utilize these reactions in synthetic chemistry.
The journey through the world of thiol attacking alkenes reveals its significance in organic synthesis and hints at vast potential waiting to be harnessed.