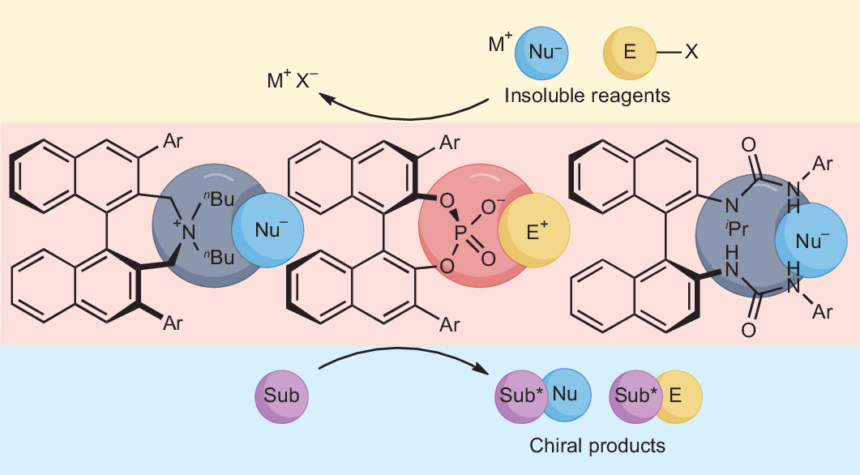
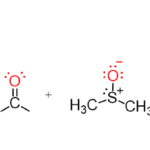
In the ever-evolving world of chemistry, certain compounds play a crucial role in enhancing reaction efficiency and selectivity. One such game-changer is the phase transfer catalyst (PTC). These remarkable substances bridge gaps between different phases—be it liquid-liquid or solid-liquid—and facilitate chemical transformations that would otherwise be sluggish or even impossible. As industries strive for greener processes and researchers seek innovative solutions, understanding PTC’s mechanisms and applications becomes paramount. Join us as we delve into the fascinating science behind phase transfer catalysts, exploring their vital importance, unique mechanisms, various types, cutting-edge innovations, real-world applications, challenges faced by practitioners in this field, and future directions for this dynamic area of research. Prepare to uncover how these catalysts are reshaping the landscape of modern chemistry!
The Importance of PTC in Chemical Reactions
Phase transfer catalysts (PTCs) play a crucial role in enhancing the efficiency of chemical reactions. They facilitate the movement of reactants between different phases, typically liquid and solid or two immiscible liquids. This increased interaction significantly speeds up reaction rates.
In various industrial processes, PTCs enable reactions that would otherwise be slow or ineffective. Their ability to bridge gaps between organic and aqueous phases allows for smoother synthesis of valuable compounds.
Without these catalysts, many synthetic pathways would remain inaccessible. This limitation can hinder product yields and increase production costs. By optimizing reaction conditions, phase transfer catalysts not only improve efficiency but also contribute to greener chemistry practices.
Moreover, their versatility means they are applicable across numerous fields, from pharmaceuticals to agrochemicals. As industries seek more sustainable solutions, PTCs stand out as vital tools in modern chemistry’s toolbox.
Mechanisms of PTC: Ion-Pair and Polymeric
Phase transfer catalysts (PTCs) operate through distinct mechanisms that enhance their effectiveness. Two primary types are the ion-pair and polymeric mechanisms.
The ion-pair mechanism involves the interaction between a cation and an anion. This pairing allows the catalyst to transport ionic species across immiscible phases. By facilitating this movement, PTCs can significantly increase reaction rates in multi-phase systems.
On the other hand, polymeric phase transfer catalysts create a more stable environment for reactions. These polymers can encapsulate reactive ions, offering both solubility and accessibility when transitioning between different phases. Their structure enhances selectivity while minimizing side reactions.
Each mechanism plays a vital role in various chemical processes, ensuring efficiency and improved yields in industrial applications. Understanding these intricate workings allows chemists to harness PTCs effectively in diverse scenarios.
Types of Phase Transfer Catalysts
Phase transfer catalysts come in various forms, each tailored for specific applications in chemical reactions.
Quaternary ammonium salts are among the most widely used types. These compounds facilitate the migration of ionic species from a polar to a nonpolar phase, effectively bridging their interactions.
Crown ethers represent another category. Their ability to encapsulate cations enhances solubility and reactivity across different phases.
Polymeric phase transfer catalysts offer unique advantages as well. They provide enhanced stability and can be reused multiple times without significant loss of efficiency.
Ionic liquids have emerged as innovative alternatives too. They can serve both as solvents and catalysts, offering unique properties that streamline many processes.
Each type plays a vital role in optimizing reaction conditions and improving yields, showcasing the versatility of phase transfer catalysts across various industries.
Innovations in PTC Technology
Innovations in phase transfer catalyst technology are reshaping the landscape of chemical synthesis. Researchers have developed novel catalysts that enhance efficiency and selectivity in reactions, reducing waste and energy consumption.
One significant advancement is the integration of nanotechnology into PTC design. Nanoparticles increase surface area and improve catalytic activity, leading to faster reaction rates. This innovation opens doors for more complex syntheses that were previously challenging.
Biodegradable and environmentally friendly PTCs are also gaining attention. These sustainable alternatives not only perform effectively but minimize environmental impact, aligning with global green chemistry initiatives.
Additionally, the application of computational modeling helps predict catalyst behavior under various conditions. By simulating reactions virtually, scientists can optimize catalyst performance before physical testing, streamlining development processes.
These innovations highlight a dynamic field where creativity meets scientific rigor, paving the way for efficient solutions in both traditional and emerging industries.
Applications of PTC in Industry and Research
Phase transfer catalysts play a crucial role across various industries by enhancing reaction efficiency. In pharmaceuticals, they facilitate the synthesis of complex compounds and intermediates, significantly reducing reaction times.
In agrochemicals, PTCs are instrumental in producing herbicides and pesticides. They allow for more effective reactions between polar and non-polar substances, which is essential for creating these vital products.
The polymer industry also benefits from phase transfer catalysts. They improve the interfacial reactions necessary for developing high-performance materials with specific properties.
Research laboratories utilize PTC to explore new chemical pathways. Their ability to bridge two immiscible phases opens up innovative avenues in synthetic chemistry.
Environmental applications are on the rise as well. Phase transfer catalysis helps in wastewater treatment processes by facilitating reactions that break down pollutants effectively.
Challenges and Limitations of PTC
Phase transfer catalysts (PTCs) are not without their challenges. One significant issue is the potential for catalyst leaching, where the PTC can escape into the product mixture, reducing its effectiveness and complicating downstream processing.
Additionally, many PTCs have specific solubility profiles. This can limit their applicability in certain solvent systems and reaction conditions. Finding a compatible solvent that allows optimal performance of the PTC often requires extensive experimentation.
Environmental concerns also arise with some traditional phase transfer catalysts. Many contain harmful or toxic components that pose risks during production and disposal. Regulatory pressures to develop greener alternatives drive researchers to innovate but add complexity to existing processes.
Scalability remains a pressing challenge. While lab-scale reactions may yield great results using a particular PTC, translating this success to industrial scales often reveals unforeseen complications in efficiency and cost-effectiveness.
Future Directions and Implications for the Chemical Industry
As the demand for greener processes grows, phase transfer catalysts are evolving. Researchers are delving into more sustainable materials that minimize environmental impact while maintaining efficiency.
Emerging technologies like machine learning and artificial intelligence could revolutionize catalyst design. Predictive models may lead to discovering novel PTCs tailored for specific reactions, enhancing performance dramatically.
Biodegradable phase transfer catalysts are gaining traction as a solution to waste disposal challenges in chemical manufacturing. These innovations promise reduced ecological footprints without sacrificing productivity.
Collaboration between academia and industry is vital. Sharing insights can accelerate advancements, ensuring that new developments meet both commercial needs and regulatory standards.
The future landscape of chemical synthesis will likely incorporate advanced PTC strategies, paving the way for safer and more efficient practices across various sectors. This shift not only benefits manufacturers but also supports global sustainability efforts.
Conclusion
Phase transfer catalysts (PTCs) have become essential tools in the field of chemistry. Their ability to facilitate reactions between immiscible phases has revolutionized many processes, making them more efficient and cost-effective. As we’ve explored, understanding their mechanisms—from ion-pair complexes to polymeric forms—offers insights into how these substances enhance reaction rates and selectivity.
Innovations in PTC technology continue to emerge, broadening their applications across various industries. Whether it’s pharmaceuticals or petrochemicals, PTCs are proving invaluable for optimizing production processes. However, challenges remain concerning scalability and environmental impact that researchers must address.
Looking forward, as advancements are made in materials science and green chemistry practices evolve, the role of phase transfer catalysts will likely expand even further. The future holds promise for new formulations that could minimize drawbacks while maximizing efficiency.
The evolution of phase transfer catalysts represents a dynamic intersection of science and industry innovation. Embracing this knowledge equips chemists with powerful tools necessary to tackle complex reactions effectively.