Why High-Fidelity Knock-In Matters
You’ve spent weeks crafting a donor template for a point mutation, optimised your guide RNA, and still your knock-in clones refuse to validate. For many labs this cycle of low integration efficiency, off-target insertions and endless screening eats up time, budgets and morale. In one survey more than half of research teams reported at least one failed knock-in project before achieving a stable line.
Many groups now turn to CRISPR gene editing — a technology that enables precise, site-specific insertions — to break this pattern. With current tools, knock-ins can often be achieved in weeks rather than months if the workflow is designed and executed carefully. High-fidelity knock-ins have moved from a specialist’s art to a repeatable process that supports reliable disease models, gene tagging and drug-target validation.
To learn more about CRISPR gene editing and how it can improve your knock-in experiments, Click here.
This article outlines practical ways to design, deliver and verify knock-ins efficiently. You’ll find research-driven insights, real-world tips and checklists you can apply immediately to reduce false starts on your next experiment.
What Is CRISPR Knock-In? Core Concepts & When to Use It
A CRISPR knock-in inserts a defined DNA sequence into a chosen genomic location using the CRISPR/Cas9 system. Unlike a knockout, which disrupts a gene, a knock-in can introduce point mutations, add epitope tags, insert fluorescent reporters or repair patient-derived mutations to create a disease model. This precision allows functional studies far beyond simple loss-of-function analysis.
Knock-in also differs from knockdown. Where knockdown only reduces expression temporarily, knock-in integrates a new sequence permanently, letting you trace proteins, test regulatory elements or correct alleles for therapeutic studies. These capabilities make it especially valuable for researchers who need high-fidelity cell models for downstream assays.
For a step-by-step overview of design strategies, donor templates and screening methods, CRISPR knock in resources provide detailed protocols and examples.
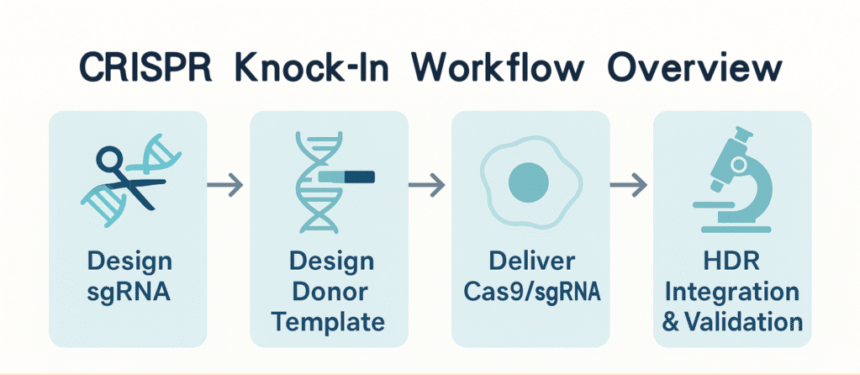
Workflow Overview with HDR Essentials
Creating a precise knock-in cell line with CRISPR is more than cutting DNA. Each step must be tuned so the cell repairs your cut with the donor template you supply. The diagram below shows the key stages of a typical knock-in workflow and highlights the repair pathway that makes it possible.
Step 1 — Design sgRNA and Donor Template
Pick a guide RNA close to your insertion site. Design the donor template with homology arms long enough to support efficient homology-directed repair (HDR), and ensure the insert does not introduce unwanted mutations or disrupt regulatory elements.
Step 2 — Delivery of CRISPR Components
Match the delivery method to your cell type: electroporation of ribonucleoprotein (RNP) complexes for high efficiency and low off-target effects, viral vectors for hard-to-transfect cells, or plasmid-based approaches for cost-effectiveness. Small pilot tests help you find the optimal balance between editing efficiency and cell viability.
Step 3 — HDR vs. NHEJ: Know Your Repair Pathway
Knock-in depends mainly on HDR, active in specific cell cycle phases. Synchronising cells or using small molecules can tilt the balance toward HDR and increase precise insertion rates. Knowing when your cells are most HDR-competent matters as much as guide design.
Step 4 — Screening and Validation
After editing, screen pooled cells for correct integration, then isolate and expand single-cell clones. Validate at both the DNA level (PCR, sequencing) and the protein level (Western blotting, immunofluorescence) to confirm the knock-in.
High-Fidelity Playbook: Parameters & Troubleshooting
Even well-designed protocols fail if key parameters are off. This playbook highlights what you can control and how to fix common problems.
Optimise Guide and Donor Design
Use validated design tools to score sgRNAs for on-target activity and off-target risk. Match homology arm length to your cell type and the size of the insert; longer arms can improve HDR for large constructs, while ssODNs work well for point mutations.
Choose the Right Delivery Strategy
Electroporation of RNP complexes often gives high efficiency and low off-target activity, but some primary or stem cells respond better to viral vectors. Test two or three options in a small pilot before scaling up.
Shift the Balance Toward HDR
Synchronise cells or use HDR-enhancing small molecules to improve precise insertion rates. Reducing Cas9 exposure after the initial cut can also limit unwanted indels.
Plan for Clones and Throughput
Pool editing can enrich for edited cells, but single-cell cloning and validation are still necessary for a stable knock-in line. Successful labs typically screen more clones than they expect.
Validate at Multiple Levels
Confirm at the DNA level with PCR and sequencing, and also verify protein expression or localisation. Use STR profiling to guard against cell line mix-ups.
Common Pitfalls and Quick Fixes
- Low HDR efficiency? Adjust homology arm design, increase donor concentration or synchronise cells.
- Random integration? Use PCR spanning junctions to confirm precise insertion and avoid misleading positives.
- False negatives? Repeat validation with independent assays; low expression doesn’t always mean no integration.
Applications that Benefit Most from Knock-In
High-fidelity knock-in cell lines solve real research problems. Three areas where precise knock-ins offer unique advantages:
Gene Tagging and Reporter Insertion
Insert epitope tags or fluorescent reporters into endogenous loci to follow proteins at native expression levels without overexpression artefacts. This improves localisation studies, protein–protein interaction mapping and live-cell imaging.
Precise Point Mutations and Disease Modelling
Recreate patient-specific mutations or correct existing ones to build robust disease models. These models help dissect the molecular basis of inherited disorders and screen therapeutic candidates under realistic conditions.
Programmable Elements and Conditional Alleles
Introduce regulatory switches, recombination sites or synthetic biology modules directly into the genome. This lets you control gene expression or create conditional alleles for sophisticated functional studies.
Case Insight: From Pilot to Validated KI Clone
A stem cell research team set out to insert a fluorescent reporter into a regulatory gene. Their first attempts with standard plasmid delivery produced low integration efficiency and many random insertions, delaying downstream assays by weeks.
They switched to a carefully planned CRISPR knock-in workflow. Two sgRNAs close to the insertion site, a donor template with long homology arms, and Cas9 delivered as an RNP via electroporation. A small pilot confirmed optimal conditions before scaling up.
Within three weeks the lab isolated several single-cell clones with the correct integration. Sequencing and Western blotting confirmed the reporter was precisely inserted and expressed at native levels, producing a reliable model for real-time gene regulation studies. This case shows that high-fidelity knock-ins depend on strategy, not luck.
Resources and Protocols for Researchers
Before you start, ask yourself:
- Is your cell line amenable to transfection or electroporation?
- Do you have a clearly defined insertion site and donor template design?
- Have you planned for enough clones and multiple validation assays?
Answering these questions early helps you decide whether to build your own cell line or work with a provider that already has the infrastructure and validated workflows. Once you have a plan, consult detailed protocols, design tools and case studies to see what has worked for other labs and what pitfalls to avoid.
High-quality guides and checklists can shorten your learning curve. For example, the CRISPR knock in resources include step-by-step strategies, donor template options and validation tips successfully applied to diverse cell types. By starting with reliable references and a clear roadmap, you increase the odds of creating a stable, reproducible knock-in cell line on your first attempt.
Conclusion: Mastering CRISPR Knock-In for Reliable Cell Models
Building a high-fidelity knock-in cell line is not a gamble; it’s a process that rewards careful design, pilot testing and rigorous validation. By applying the principles in this guide — optimising sgRNA and donor design, matching delivery to cell type, shifting the balance toward HDR and validating at multiple levels — you can transform a high-risk experiment into a predictable workflow.
CRISPR technology now makes it possible to introduce precise, heritable changes in a fraction of the time required by older methods. Used strategically, it enables researchers to create disease models, tag genes, correct mutations and insert programmable elements with unprecedented accuracy.
As you plan your next project, start with a clear roadmap, consult reliable protocols and build in time for small-scale testing and thorough screening. This research-driven approach will increase your success rate and shorten the path from concept to validated cell model — turning CRISPR knock-in from a buzzword into an everyday tool in your lab.